MB Research - In Vitro Sensitization ITS
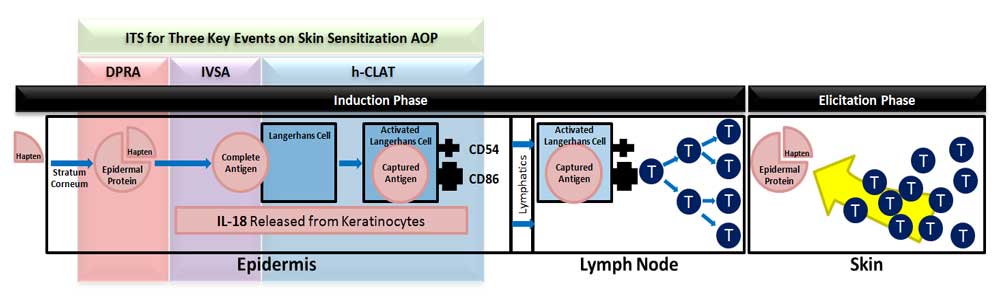
MB Research has developed a non-animal Integrated Testing Strategy (ITS) for chemical-induced contact hypersensitivity
(skin sensitization). This ITS provides a completely non-animal
alternative to traditional testing methods. Together, our three assays address three key events on the skin
sensitization adverse outcome pathway (AOP).
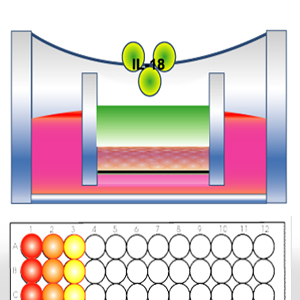
The In Vitro Sensitization Assay (IVSA) is a keratinocyte
activation test. IVSA uses an ELISA method to measure IL-18 release from a topically treated reconstructed 3D human
keratinocyte tissue model.
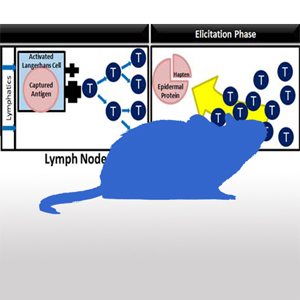
The Human Cell Line Activation Test (h-CLAT) is a dendritic
cell activation test. The h-CLAT uses flow cytometry to measure CD86 and CD54 expression on treated THP-1 cells in culture.
The Direct Peptide Reactivity Assay (DPRA) is an in chemico
method used to predict epidermal protein binding. Binding of epidermal proteins is the molecular initiating event on the AOP.
The DPRA uses HPLC to measure the depletion of synthetic peptides in solution following exposure to test chemicals.