MB Research Labs has been developing a novel assay to determine the potential of a 3D reconstructed human epidermal (RHE) tissue that is co-cultured with human plasmacytoid Dendritic Cells (pDCs) for use as an in vitro dermal sensitization test.
Two is better than one
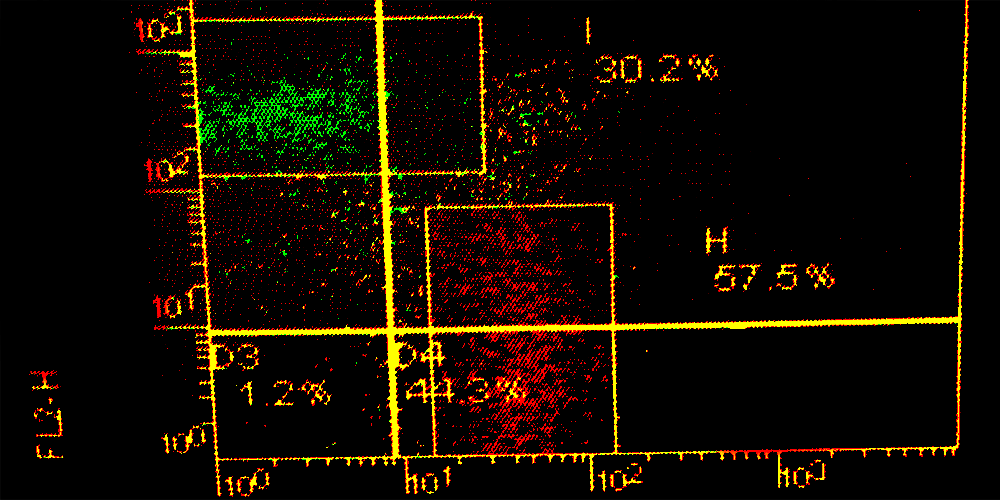
In this combined assay, RHE tissues are placed at the air-liquid interface above a media suspension of pDC. The tissues are then exposed to test materials, and after a short period of incubation, the RHE tissues and pDC were separately cultured for an additional 20 hours. The RHE media was analyzed for IL-18 release by ELISA, and the pDC were analyzed for changes in CD86 surface expression by flow cytometry. Two non-sensitizing irritants (Lactic Acid and Phenol), along with two weak/moderate sensitizers: Eugenol and Hexylcinnamaldehyde, and two strong sensitizers: 1-Chloro-2,4-Dinitrobenzene and 4-Nitrobenzyl Bromide were assayed. A positive response from the RHE tissues was determined to be a 2-fold increase in IL-18 secretion, and a 1.5 fold increase in CD86 expression on pDC. Tissue viability was measured using the MTT assay.
Consistent Responses
The responses we obtained in both the RHE tissue versus pDC were very consistent. Increases in both secretion of IL-18 and expression of CD86 were detected after exposure to dermal sensitizers. A prediction model was developed in which a sensitizer result for a chemical is defined as either a positive result in the RHE tissue (IL-18) or a positive result in pDCs (CD86). From three individual experiments, and using a 2×2 contingency table to determine Cooper statistics, we obtained an Accuracy of 100%, 83%, and 83% (89% mean Accuracy). All four of four sensitizers were positively predicted in each experiment (100% Sensitivity).
Conclusions
We hypothesized that co-culturing a 3D RHE skin model and pDC model would allow communication between the skin-like tissues and human-derived pDC and that interaction may allow for a more complete assessment of delayed-type hypersensitivity immune reactions of the measured endpoints yielding a more accurate in vitro sensitization assay. Based upon our on-going research, we have established the following conclusions:
- An advantage of this Co-Culture test system is that sensitization responses to both keratinocytes (IL-18) and pDC (CD86) can be assessed concurrently.
- RHE tissues allow for topical application of products in many chemical forms, such as liquids, solids, gels, lotions and powders.
- Although the stronger sensitizers NBB and DNCB secrete IL-18 between 4 and 24 hours after dosing, the weaker sensitizer Eugenol secreted higher amounts of IL-18 within 4 hours.
- From three independent experiments we obtained a mean accuracy of 89%. Future directions will include testing of additional irritants, sensitizers, mixtures and commercial products.
Note: This research was funded by the Society of Toxicology Grant for Alternatives Research (sponsored by Colgate-Palmolive).
For more information about this and other in vitro toxicology assays, please see: www.mbresearch.com