human Cell Line Activation Test
(h-CLAT) OECD 442E
Acute Dermal Sensitization is a key safety endpoint the evaluation of human skin sensitization potential. Traditionally, animal tests like the Guinea Pig Maximization Test and Buehler Test have been used to assess dermal sensitization, as well as the murine Local Lymph Node Assay (LLNA). In vitro assays are now developed for skin sensitization screening using an Adverse Outcome Pathway (AOP) focusing on the first three key events of the induction phase: Protein Binding, Keratinocyte Activation and Dendritic Cell Activation.
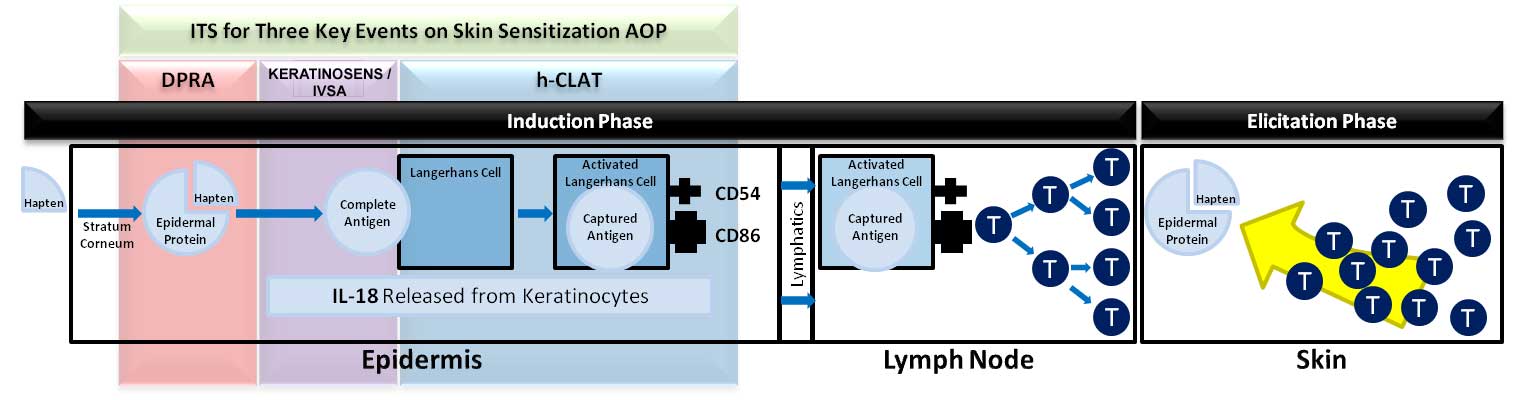
The in vitro skin sensitization test – human Cell Line Activation Test (h-CLAT), was developed as a Dendritic Cell or Langerhans cell (LC) Activation model for dermal sensitization and uses the THP-1 cell line, a human monocytic leukemia cell line, as an LC surrogate. In the h-CLAT, the augmentation of CD86 and CD54 expression in THP-1 cells is measured by flow cytometry following a 24-hr exposure to a test substance. The h-CLAT is a complicated assay with many facets that require precision and attention to detail. Herein we outline and address some pitfalls, issues and remediation in the h-CLAT procedures.
Tripping over Cell Line Issues:
Proactive procedures should be made to bank frozen cell stocks at various passages. Historical data on the doubling time and passage number should be maintained and monitored.
Reactivity Checks:
The reactivity check, which is performed two weeks after thawing a new batch of THP-1 cells, requires ≥90% viability of cells treated with the vehicle control and those treated with lactic acid (LA). Positive controls: 2,4 dinitrochlorobenzene (DNCB) and nickel sulfate (NiSO4) should produce a positive response in CD86 and CD54 expression, and LA should produce a negative response. If these requirements are not met, remedial steps should be taken, including possibly thawing a new batch of cells.
Test Substance Solubility:
The solubility of the test substance can also be problematic and should be evaluated and confirmed visually. An appropriate solvent (saline, culture medium, or dimethyl sulfoxide [DMSO]) should completely dissolve the test substance. A reliable CV75 should be derived from at least two independent screens; however, if there is difficulty attaining this, alternative vehicles – chosen with scientific justification – can be qualified to broaden the applicability domain.
Flow Cytometric Analysis:
Two important flow cytometry h-CLAT tips are keeping the preparation of FACS buffer and 1% globulin ice cold (~4°C) and ensuring you have high quality maintenance of the flow cytometer, which is essential for reliable data acquisition.
h-CLAT Testing:
MB Research Labs has over 20 years of experience in sensitization testing using guinea pig, mouse and cell culture test methods. Our dedicated team of toxicologists who have extensive experience in performing the h-CLAT as well as other in vitro and in vivo dermal sensitization tests would be happy to discuss your sensitization testing needs and assess which appropriate test method can suit your testing requirements.
For more information: www.mbresearch.com/contact.htm